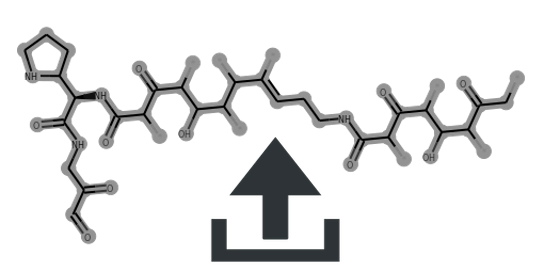
Cluster scaffolds:
1 |
Putative metabolites:
| Name | Rank | Metabolite Score | Similarity | MCS Score | Post-Mod Score | Post-Mod Info | Source | Link | Smiles | Molview | Full Name | Figure | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| pactamycin | 1 | 0.41 | 0.49 | 0.46 | 0.0 | Glyco:0/3 * 6-Ring:0/1 | MIBiG | Source | CC(=O)c1cccc(N[C@H]2[C@H](N)[C@@](NC(=O)N(C)C)([C@H](C)O)[C@@](C)(O)[C@@]2(O)COC(=O)c2c(C)cccc2O)c1 | view | pactamycin | ||
| Xenortide C | 2 | 0.33 | 0.49 | 0.29 | 0.0 | Glyco:0/3 * 6-Ring:0/1 | MIBiG | Source | CN[C@H](C(=O)N(C)[C@@H](Cc1ccccc1)C(=O)NCCc1ccccc1)C(C)C | view | Xenortide C | ||
| Xenortide A | 3 | 0.32 | 0.49 | 0.28 | 0.0 | Glyco:0/3 * 6-Ring:0/1 | MIBiG | Source | CN[C@@H](CC(C)C)C(=O)N(C)[C@@H](Cc1ccccc1)C(=O)NCCc1ccccc1 | view | Xenortide A | ||
| pacidamycin 5 | 4 | 0.3 | 0.48 | 0.23 | 0.0 | Glyco:0/3 * 6-Ring:0/1 | MIBiG | Source | C[C@H](NC(=O)N[C@@H](Cc1ccccc1)C(=O)O)C(=O)N[C@H](C(=O)N/C=C1/C[C@@H](O)[C@H](n2ccc(=O)[nH]c2=O)O1)[C@H](C)N(C)C(=O)[C@@H](N)Cc1cccc(O)c1 | view | pacidamycin 5 | ||
| Hapalosin | 0 | 0.44 | 0.49 | 0.52 | 0.0 | Glyco:0/3 * 6-Ring:0/1 | MIBiG | Source | CCCCCCC[C@H]1OC(=O)C[C@@H](O)[C@H](Cc2ccccc2)N(C)C(=O)[C@H](C(C)C)OC(=O)[C@H]1C | view | Hapalosin |