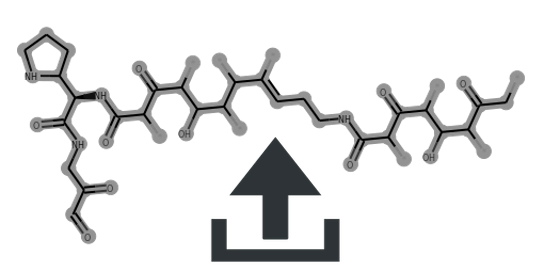
Cluster scaffolds:
1 |
Putative metabolites:
| Name | Rank | Metabolite Score | Similarity | MCS Score | Post-Mod Score | Post-Mod Info | Source | Link | Smiles | Molview | Full Name | Figure | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| pactamycin | 0 | 0.51 | 0.49 | 0.41 | 1.0 | Non detected. | MIBiG | Source | CC(=O)c1cccc(N[C@H]2[C@H](N)[C@@](NC(=O)N(C)C)([C@H](C)O)[C@@](C)(O)[C@@]2(O)COC(=O)c2c(C)cccc2O)c1 | view | pactamycin | ||
| heterobactin A | 1 | 0.5 | 0.53 | 0.35 | 1.0 | Non detected. | MIBiG | Source | N/C(=N\C(=O)c1cccc(O)c1O)NCCC[C@@H](NC(=O)c1cccc(O)c1O)C(=O)NCC(=O)N[C@H]1CCCN(O)C1=O | view | heterobactin A | ||
| heterobactin S2 | 2 | 0.49 | 0.53 | 0.34 | 1.0 | Non detected. | MIBiG | Source | N/C(=N\C(=O)c1cccc(O)c1O)NCCC[C@@H](NC(=O)c1ccc(S(=O)(=O)O)c(O)c1O)C(=O)NCC(=O)N[C@H]1CCCN(O)C1=O | view | heterobactin S2 | ||
| Desmethylsalinamide E | 3 | 0.46 | 0.49 | 0.3 | 1.0 | Non detected. | MIBiG | Source | CC[C@H](C)[C@H]1NC(=O)[C@@H](NC(=O)[C@H](C)[C@H](O)C(C)C)[C@@H](C)OC(=O)[C@H](CO)NC(=O)[C@@H]([C@@H](C)O)NC(=O)[C@H](Cc2ccccc2)N(C)C(=O)[C@H](c2ccc(O)cc2)NC1=O | view | Desmethylsalinamide E | ||
| cahuitamycin B | 4 | 0.46 | 0.51 | 0.29 | 1.0 | Non detected. | MIBiG | Source | O=CN(O)CCC[C@@H](NC(=O)[C@H](CO)NC(=O)[C@H](CO)NC(=O)c1ccccc1O)C(=O)N1NCCC[C@@H]1C(=O)NCCC(=O)O | view | cahuitamycin B |