Cluster scaffolds:
1 |
Putative metabolites:
| Name | Rank | Metabolite Score | Similarity | MCS Score | Post-Mod Score | Post-Mod Info | Source | Link | Smiles | Molview | Full Name | Figure | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
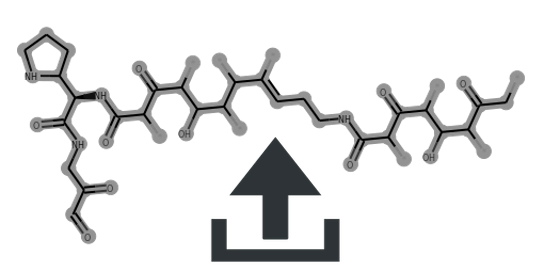
| meridamycin | 0 | 0.68 | 0.75 | 0.7 | 0.4 | Glyco:0/3 * 6-Ring:1/1 | MIBiG | Source | CC/C1=C/C[C@@H](/C(C)=C/[C@@H](C)[C@@H](C)O)OC(=O)C2CCCCN2C(=O)C(=O)[C@]2(O)O[C@@H](CC[C@H]2C)C[C@H](O)[C@H](C)[C@@H](O)C[C@@H](O)C[C@H](O)/C(C)=C\[C@@H](C)CC(C)[C@@H]1O | view | meridamycin | ||
| halstoctacosanolide | 1 | 0.62 | 0.59 | 0.69 | 0.4 | Glyco:0/3 * 6-Ring:1/1 | MIBiG | Source | CCC(O)C(C)C(O)CC(O)C(O)/C=C(\C)CC(C)C1CC(O)C(C)/C=C\C=C/CC(C)C(=O)CC2CCC(C)C(O)(C/C=C(C)\C=C(\C)C(O)C(=O)/C=C(/C)C(=O)O1)O2 | view | halstoctacosanolide | ||
| brasilinolide C | 2 | 0.62 | 0.55 | 0.66 | 0.67 | Glyco:1/3 * 6-Ring:1/1 | MIBiG | Source | C[C@@H]1O[C@@H](O[C@@H](C)[C@H](C)[C@H](O)[C@@H](C)[C@H](O)[C@H](C)[C@H]2OC(=O)/C=C\C[C@H](O)C[C@H](O)C[C@@H](O)CC[C@@H](C)[C@H](O)C[C@]3(O)O[C@H](C[C@@H](O)C[C@H](O)CCC[C@H](O)[C@@H]4O[C@H]4[C@@H]2C)C[C@H](O)[C@H]3O)C[C@H](O)[C@@H]1O | view | brasilinolide C | ||
| filipin | 3 | 0.58 | 0.57 | 0.74 | 0.0 | Glyco:0/3 * 6-Ring:0/1 | MIBiG | Source | CCCCC[C@@H](O)[C@H]1C(=O)O[C@H](C)[C@@H](O)/C=C\C=C/C=C\C=C/C=C(/C)[C@@H](O)C[C@H](O)C[C@H](O)C[C@H](O)C[C@H](O)C[C@H](O)C[C@@H]1O | view | filipin | ||
| mycolactone | 4 | 0.44 | 0.55 | 0.46 | 0.0 | Glyco:0/3 * 6-Ring:0/1 | MIBiG | Source | C/C1=C/C[C@H]([C@@H](C)C/C(C)=C/[C@@H](C)[C@H](O)C[C@@H](C)O)OC(=O)CCC[C@H](OC(=O)/C=C/C(C)=C/C(C)=C/C=C/C(C)=C/[C@H](O)[C@@H](O)C[C@H](C)O)[C@@H](C)C1 | view | mycolactone |