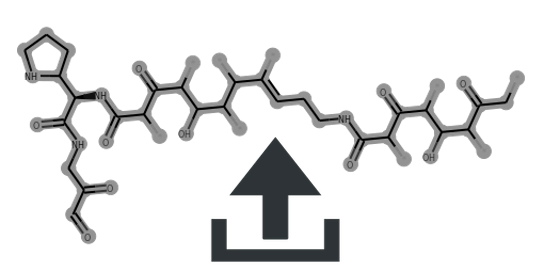
Cluster scaffolds:
1 |
Putative metabolites:
| Name | Rank | Metabolite Score | Similarity | MCS Score | Post-Mod Score | Post-Mod Info | Source | Link | Smiles | Molview | Full Name | Figure | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| candicidin | 1 | 0.55 | 0.54 | 0.59 | 0.4 | Glyco:1/3 * 6-Ring:0/1 | MIBiG | Source | CC1/C=C\C=C/C=C\C=C/C=C\C=C/C=C\C(OC2OC(C)C(O)C(N)C2O)CC(O)C(C(=O)O)C(O)CC(=O)CC(O)CC(O)CC(O)CC(=O)CCCC(=O)CC(=O)OC1C(C)CC(C)C(O)CC(=O)c1ccc(N)cc1 | view | candicidin | ||
| nystatin A1 | 2 | 0.55 | 0.53 | 0.54 | 0.67 | Glyco:1/3 * 6-Ring:1/1 | MIBiG | Source | C[C@@H]1OC(=O)C[C@H](O)C[C@H](O)C[C@H](O)CC[C@@H](O)[C@H](O)C[C@]2(O)C[C@H](O)[C@@H](C(=O)O)[C@H](C[C@@H](O[C@@H]3O[C@H](C)[C@@H](O)[C@H](N)[C@@H]3O)/C=C\C=C/C=C\C=C/CC/C=C\C=C/[C@H](C)[C@@H](O)[C@H]1C)O2 | view | nystatin A1 | ||
| Amphotericin B | 3 | 0.54 | 0.53 | 0.52 | 0.67 | Glyco:1/3 * 6-Ring:1/1 | MIBiG | Source | CC1OC(O[C@H]2/C=C\C=C/C=C\C=C/C=C\C=C/C=C\[C@H](C)[C@@H](O)[C@@H](C)[C@H](C)OC(=O)C[C@H](O)C[C@H](O)CC[C@@H](O)[C@H](O)C[C@H](O)C[C@]3(O)C[C@H](O)[C@@H](C(=O)O)[C@H](C2)O3)C(O)C(N)C1O | view | Amphotericin B | ||
| Etnangien | 4 | 0.53 | 0.53 | 0.66 | 0.0 | Glyco:0/3 * 6-Ring:0/1 | MIBiG | Source | COC1C(C)C(O)CC(=O)OC(C(C)C(O)/C=C(C)/C=C/C=C/C=C/C=C/C=C/CC(O)/C=C(\C)CCC(=O)O)CC(O)CCCC(O)C/C=C\C=C/CC(C)C(O)C1C | view | Etnangien | ||
| ECO-02301 | 0 | 0.6 | 0.62 | 0.63 | 0.4 | Glyco:1/3 * 6-Ring:0/1 | MIBiG | Source | CC(/C=C/CC/C=C/C=C/C=C/C=C/C(=O)NC1=C(O)CCC1=O)C(O)C(C)C(O)/C=C/C=C/C=C/C=C/C=C/CC(O[C@@H]1O[C@@H](C)[C@H](O)[C@@H](O)[C@H]1O)C(C)C(=O)CC(O)CC(O)CC(O)/C=C/CC(O)CC(O)CC(O)CC(O)/C=C/CC(O)CC(O)CCCN | view | ECO-02301 |