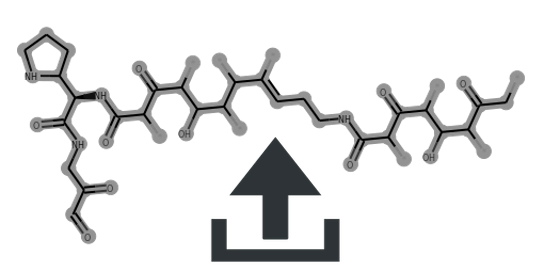
Cluster scaffolds:
1 |
2 |
Putative metabolites:
| Name | Rank | Metabolite Score | Similarity | MCS Score | Post-Mod Score | Post-Mod Info | Source | Link | Smiles | Molview | Full Name | Figure | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| tyrocidine | 0 | 0.71 | 0.59 | 0.72 | 1.0 | Non detected. | MIBiG | Source | CC(C)C[C@@H]1NC(=O)[C@H](CCCN)NC(=O)[C@H](C(C)C)NC(=O)[C@H](Cc2ccc(O)cc2)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@H](CC(N)=O)NC(=O)[C@@H](Cc2ccccc2)NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@@H]2CCCN2C(=O)[C@@H](Cc2ccccc2)NC1=O | view | tyrocidine | ||
| cyclomarin D | 1 | 0.66 | 0.59 | 0.63 | 1.0 | Non detected. | MIBiG | Source | C=CC(C)(C)n1cc([C@@H](O)[C@@H]2NC(=O)[C@H]([C@H](C)C=C(C)C)NC(=O)[C@H](CC(C)C)N(C)C(=O)[C@H](C(C)C)NC(=O)[C@H]([C@H](OC)c3ccccc3)NC(=O)[C@H](C)NC(=O)[C@H](C[C@@H](C)CO)NC2=O)c2ccccc21 | view | cyclomarin D | ||
| Desmethylsalinamide C | 2 | 0.66 | 0.61 | 0.61 | 1.0 | Non detected. | MIBiG | Source | C/C=C(C)/C=C/C(=O)NCC(=O)OC[C@@H]1NC(=O)[C@@H]([C@@H](C)O)NC(=O)[C@H](Cc2ccccc2)N(C)C(=O)[C@@H](c2ccc(O)cc2)NC(=O)[C@@H]([C@@H](C)CC)NC(=O)[C@@H](NC(=O)[C@H](C)[C@H](O)C(C)C)[C@@H](C)OC1=O | view | Desmethylsalinamide C | ||
| Salinamide F | 3 | 0.65 | 0.59 | 0.6 | 1.0 | Non detected. | MIBiG | Source | CC[C@H](C)[C@H]1NC(=O)[C@@H](NC(=O)[C@H](C)[C@H](O)C(C)C)[C@@H](C)OC(=O)[C@@H]2COC(=O)CNC(=O)/C=C\[C@](O)(CO)[C@@H](C)Oc3ccc(cc3)[C@H](NC1=O)C(=O)N(C)[C@@H](Cc1ccccc1)C(=O)N[C@H]([C@@H](C)O)C(=O)N2 | view | Salinamide F | ||
| nostamide A | 4 | 0.64 | 0.59 | 0.59 | 1.0 | Non detected. | MIBiG | Source | CC[C@@H](C)[C@@H]1NC(=O)[C@H](NC(=O)N[C@@H](Cc2ccccc2)C(=O)O)CCCCNC(=O)[C@H](CCc2ccc(O)cc2)NC(=O)CN(C)C(=O)[C@H](CCc2ccccc2)NC1=O | view | nostamide A |