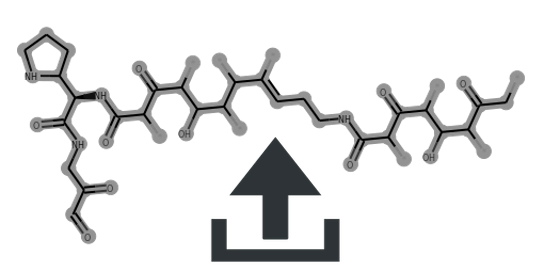
Cluster scaffolds:
1 |
2 |
Putative metabolites:
| Name | Rank | Metabolite Score | Similarity | MCS Score | Post-Mod Score | Post-Mod Info | Source | Link | Smiles | Molview | Full Name | Figure | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sevadicin | 0 | 0.57 | 0.59 | 0.7 | 0.0 | Glyco:0/3 | MIBiG | Source | CC(NC(=O)C(N)Cc1ccccc1)C(=O)NC(Cc1c[nH]c2ccccc12)C(=O)O | view | Sevadicin | ||
| Microsclerodermin M | 1 | 0.47 | 0.57 | 0.52 | 0.0 | Glyco:0/3 | MIBiG | Source | CN1CC(=O)N[C@H](Cc2c[nH]c3ccccc23)C(=O)NCC(=O)NC[C@H](O)CC(=O)N[C@H]([C@H](O)[C@@H](O)C/C=C/C=C/C=C/c2ccccc2)[C@H](O)C(=O)N[C@@H]2CC(=O)N[C@@]2(O)CC1=O | view | Microsclerodermin M | ||
| pacidamycin 4 | 2 | 0.46 | 0.58 | 0.48 | 0.0 | Glyco:0/3 | MIBiG | Source | C[C@H](NC(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)O)C(=O)N[C@H](C(=O)N/C=C1/C[C@@H](O)[C@H](n2ccc(=O)[nH]c2=O)O1)[C@H](C)N(C)C(=O)[C@@H](N)Cc1cccc(O)c1 | view | pacidamycin 4 | ||
| pacidamycin 1 | 3 | 0.46 | 0.61 | 0.46 | 0.0 | Glyco:0/3 | MIBiG | Source | C[C@H](N)C(=O)N[C@@H](Cc1cccc(O)c1)C(=O)N(C)[C@@H](C)[C@H](NC(=O)[C@H](C)NC(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)O)C(=O)N/C=C1/C[C@@H](O)[C@H](n2ccc(=O)[nH]c2=O)O1 | view | pacidamycin 1 | ||
| pacidamycin 6 | 4 | 0.45 | 0.6 | 0.46 | 0.0 | Glyco:0/3 | MIBiG | Source | C[C@H](NC(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)O)C(=O)N[C@H](C(=O)N/C=C1/C[C@@H](O)[C@H](n2ccc(=O)[nH]c2=O)O1)[C@H](C)N(C)C(=O)[C@H](Cc1cccc(O)c1)NC(=O)CN | view | pacidamycin 6 |